Answer : The bond dissociation energy is 1656 kJ/mol
Explanation :
In methane, the energy required to break the one C-H bond is 414 kJ/mol.
Now we have to determine the bond dissociation energy for the breaking of all the bonds in a mole of methane.
The formula of methane is,

In methane, there are 4 number of C-H bonds. So, the bond dissociation energy
 will be:
will be:
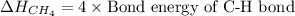
Now put the value of bond energy of C-H bond in this expression, we get:
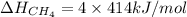
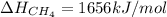
Hence, the bond dissociation energy is 1656 kJ/mol