Answer:
To supply the required ions it is necessary to inject 5,6mL of 6g/30mL solution and 131,1 mL of 0,9% solution.
Step-by-step explanation:
1mEq of sodium are 59mg of NaCl and 1mEq of potassium are 75mg KCl
in intravenous infusion 15 mEq of K are:
15x75mg KCl = 1,125g of KCl
And 20 mEq of Na are:
20x59mg NaCl = 1,18g of NaCl
To supply the potassium ion it is necessary to inject:
1,125g of KCl×
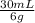 = 5,6mL of 6g/30mL solution
= 5,6mL of 6g/30mL solution
And, to supply the sodium ion it is necessary to inject:
1,18g of NaCl×
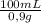 = 131,1 mL of 0,9% solution
= 131,1 mL of 0,9% solution
I hope it helps!