Answer:
Solubility will be = 1.24 X 10⁻⁴ M
Step-by-step explanation:
When the concentration of the salt dissolved in water is very low the concentration of hydroxide ions generated by the water can be ignored.
The dissociation of metal hydroxide can be expressed as:
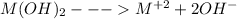
Ksp =
![[M^(+2)][OH^(-)]^(2)](https://img.qammunity.org/2020/formulas/chemistry/college/uy5b987spfsah7i028jiuwpxjef7envyo0.png)
Let the solubility of the metal hydroxide is "s"
Ksp = (s)(2s)²=4s³
Ksp = 7.65X10⁻¹² = 4s³
Therefore
s = 1.24 X 10⁻⁴ M