Answer:
The answer to your question is: 0.02 M
Step-by-step explanation:
Data
mass = 5.57 g
volume = 2.50 L
Formula
Molarity =
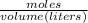
Process
1.- Calculate the molecular mass of CaCl₂
Molecular mass 0f CaCl₂ = 40 + (35.5 x 2) = 111 g
2.- Calculate the moles of CaCl₂ in 5.57 g
111 g of CaCl₂ ---------------- 1 mol
5.57 g of CaCl₂ ------------- x
x = (5.57 x 1) / 111
x = 0.050 moles of CaCl₂
3.- Calculate molarity
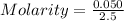
Molarity = 0.02