Answer: Option (d) is the correct answer.
Step-by-step explanation:
According to the given situation, mass of compound will be calculated as follows.
Mass of compound = mass of flask and condensed vapor - mass of flask
= 115.23 - 114.85
= 0.38 g
Volume (V) = 255 mL =
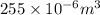 (as 1 ml =
(as 1 ml =
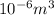 )
)
Pressure (P) = 101325 Pa
Temperature =
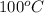 = (100 + 273) K = 373 K
= (100 + 273) K = 373 K
Now, according to the ideal gas equation, PV = nRT
and, moles of compound n =
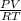
=
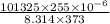
= 0.008332 mol
As, molar mass of compound =
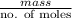
=
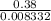
= 46 g/mol
Therefore, the compound is
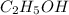 (molar mass = 12 x 2 + 5 x 1 + 16 + 1 = 46 g/mol).
(molar mass = 12 x 2 + 5 x 1 + 16 + 1 = 46 g/mol).
Thus, we can conclude that out of the given options the liquid could be
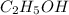 .
.