Answer:
1)Qa=34956 MJ
2)COP=7.51
COP= 24.5
Step-by-step explanation:
Given that
Qr= 120,000 KJ/h
We know that
1 day = 24 hr
14 day =24 x 14 hr
14 day = 336 hr
Qr= 120000 x 336 KJ
Qr= 40320 MJ
W= 1490 KW.h ( 1 h = 3600 s)
W= 5364 MJ
We know that
From first law of thermodynamics
Qr= Qa+W
Qa= 40320 - 5364 MJ
1)Qa=34956 MJ
COP = Qr/W
COP= 40320 / 5364
2)COP=7.51
3)COP of ideal heat pump
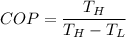
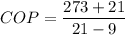
COP= 24.5