Answer: The correct answer is Option B.
Step-by-step explanation:
Double displacement reaction is defined as the chemical reaction in which exchange of ions takes place.
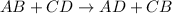
An insoluble salt in a solution is termed as a precipitate.
When an aqueous solution of magnesium nitrate reacts with an aqueous solution of potassium carbonate, it leads to the formation of an aqueous solution of potassium nitrate and a solid precipitate of magnesium carbonate.
The chemical equation for the above reaction follows:
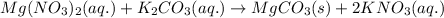
Hence, the correct answer is Option B.