Answer: The pH of the lake after accident is 5.44
Step-by-step explanation:
To calculate the molarity of lake, we use the equation:

where,
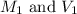 are the molarity and volume of the water
are the molarity and volume of the water
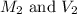 are the molarity and volume of hydrochloric acid
are the molarity and volume of hydrochloric acid
We are given:
Conversion factor used:
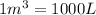
![M_1=?M\\V_1=(5* 10^5m^3+150L)=[(5* 10^8)+(150)]L\\M_2=12.0M\\V_2=150L](https://img.qammunity.org/2020/formulas/chemistry/high-school/etudhcs6a751r70xiw9nbi5qfijjm5kol1.png)
Putting values in above equation, we get:
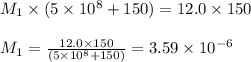
To calculate the pH of the solution, we use the equation:
![pH=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vz65x0ueuj8r8ibqa81zvsbzb2yaetlce4.png)
We are given:
![[H^+]=3.59* 10^(-6)](https://img.qammunity.org/2020/formulas/chemistry/high-school/2qwq5vf993ofpwe1sutux58a704qngpgrp.png)
Putting values in above equation, we get:
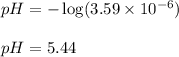
Hence, the pH of the lake after accident is 5.44