Answer:
The correct answer is option D.
Step-by-step explanation:
Mass of compound = 42.0 g
Mass of carbon in compound = 36.0 g
Mass of hydrogen in compound = 6.0 g
Moles of carbon =
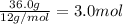
Moles of hydrogen =
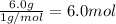
Empirical formula of the compound, divide least number of moles from each element.
Carbon =
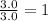
Hydrogen =
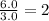
Empirical formula of compound =
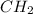
The empirical formula of the compound can be calculated from the given data.