Answer:
The Dipole moment is 6.77 Debyes.
Step-by-step explanation:
Given bond length of HBr molecule = 1.41 A°,
We also know charges on H and Br(+1 and -1 respectively)
Charge on H = (+1)
 (absolute charge of electron);
(absolute charge of electron);
Charge on Br = (-1)
 (absolute charge of electron);
(absolute charge of electron);
Since absolute charge on electron =
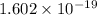 ;
;
Therefore charge on H =
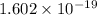 Coulomb;
Coulomb;
charge on Br =
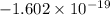
We know Dipole Moment = Charge
 Distance;
Distance;
Here,
Distance = Bond Length = 1.41 A° = 1.41
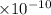 ;
;
and charge on atom =
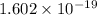 Coulomb;
Coulomb;
Therefore,
Dipole Moment = Distance
 Charge
Charge
Dipole Moment =
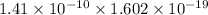 ;
;
Dipole Moment =
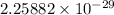 SI units.
SI units.
The conversion factor of for SI units to Debye is 2.9989
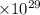
Therefore,
Dipole Moment = 2.25882
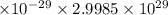
Dipole Moment = 6.77 Debye.