Answer:
a.36 g of water is produced.
b.64 g of
 is consumed.
is consumed.
Step-by-step explanation:
The reaction is
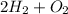 ⇒
⇒
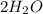
a.
Given,
Weight of
 reacted = 4g
reacted = 4g
Weight of 1 mole of
 = 2
= 2
 1 = 2g
1 = 2g
Therefore no. of moles of
 reacted =
reacted =
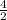 = 2 moles;
= 2 moles;
Also given,
Weight of
 reacted = 32 g
reacted = 32 g
Weight of 1 mole of
 = 2
= 2
 16 = 32 g
16 = 32 g
Therefore no. of moles of
 reacted =
reacted =
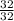 = 1
= 1
We know that 2 moles of Hydrogen reacts with 1 mole of Oxygen to give 2 moles of water,
As we took 2 moles of Hydrogen and 1 mole of Oxygen,
Directly,from the equation we can tell 2moles of water will be produced.
Therefore no. of moles of
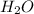 produced = 2
produced = 2
Weight of 1 mole of water =
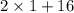 = 18
= 18
Therefore weight of
 produced =
produced =
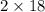 = 36gm
= 36gm
b.
Given ,
72 g of
 is produced.
is produced.
So,
no. of moles of
 produced =
produced =
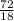 = 4 moles
= 4 moles
From equation For every 2 moles of water formed , 1 mole of oxygen must be required.
So for producing 4 moles of water,
No. of moles of Oxygen required = 2 moles.
Therefore weight of
 reacted =
reacted =
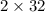 = 64 g
= 64 g
Method 2:
Given,
8 g of
 has reacted.
has reacted.
So,
no. of moles of
 reacted =
reacted =
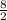 = 4 moles.
= 4 moles.
From equation , we know that For every 2 moles of
 reacted,1 mole of
reacted,1 mole of
 will react.
will react.
Therefore,
No. of moles of
 that reacts with 4 moles of
that reacts with 4 moles of
 =
=
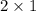 = 2 moles
= 2 moles
Therefore the weight of
 reacted =
reacted =
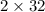 = 64 g
= 64 g