Answer:
ΔH = -98 kJ/mol
Step-by-step explanation:
To calculate the heat change of the reaction:
HCl(aq) + NH₃(aq) → NH₄Cl(aq)
0.2M 0.2M ΔT=2.34°C
1x10²mL 1x10²mL
We need to use the next equation:
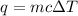 (1)
(1)
where q: the amount of heat energy lost or gained, m: the mass of the substance, c: the specific heat capacity of the substance and ΔT: the change in temperature of the substance
Assuming that the densities of the solutions are the same as for water, we can determine the mass of the solution:
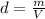
where d: density, m: mass and V: volume of solution = 100 + 100 = 200mL
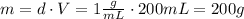
Now, using the calculated mass in equation (1), and assuming that the specific heats of the solutions are the same as for water, we can find heat change of the reaction:
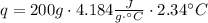
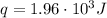
This heat is negative because is the heat lost by the reacting HCl and NH₃ and gained by the water, so:
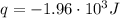
To calculate the heat change of the reaction per mole of HCl, we need to divide the heat change by the number of moles, which is called the enthalpy of reaction:
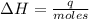
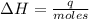
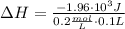
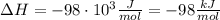
So, the heat change of the reaction per mole of HCl reacted, often called enthalpy of reaction, is ΔH = -98 kJ/mol.
Have a nice day!