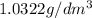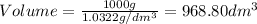Answer :
(a) The value of volume of air at temperature
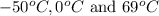 are
are
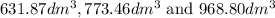 respectively.
respectively.
Explanation :
(a) The formula used for density is:

or,
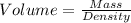
Now we have to calculate the volume at each temperature.
The mass of sample = 1000 g
At temperature
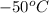 :
:
Density =
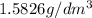
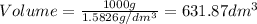
At temperature
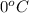 :
:
Density =
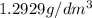
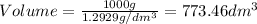
At temperature
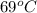 :
:
Density =