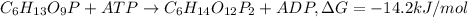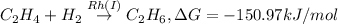Answer : The two spontaneous chemical reactions are:
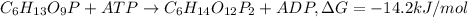
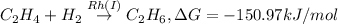
Explanation :
Gibbs free energy : It is defined as the amount of energy that is available to do useful work.
A reaction to be spontaneous when
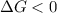
A reaction to be non-spontaneous when
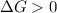
For the reaction to be spontaneous, the Gibbs free energy of the reaction
 is negative or we can say that the value of
is negative or we can say that the value of
 is less than zero.
is less than zero.
From the given chemical reactions we conclude that there are two reactions that are spontaneous (favorable).
The two spontaneous chemical reactions are: