Answer : The volume of the balloon after 45.0 hour is 128.5 L
Explanation :
First we have to calculate the moles of helium leaked.
In 1 hour, the number of moles of helium leaked = 10.0 mmol
In 45.0 hour, the number of moles of helium leaked =
![\frac{45.0hr]{1hr}* 10.0=450mmol=0.450mole]()
conversion used : 1 mmol = 0.001 mole
Now we have to calculate the number of moles of helium remaining in the balloon.
Moles of helium remaining in the balloon = Total moles of helium - Moles of helium leaked
Moles of helium remaining in the balloon = 5.50 - 0.450 = 5.05 mole
Now we have to calculate the volume of the balloon after 45.0 hour.
According to the Avogadro's law, the volume of gas is directly proportional to the number of moles of gas at same pressure and temperature. That means,
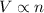
or,
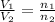
where,
 = initial volume of gas = 140.0 L
= initial volume of gas = 140.0 L
 = final volume of gas = ?
= final volume of gas = ?
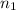 = initial moles of gas = 5.50 mole
= initial moles of gas = 5.50 mole
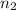 = final moles of gas = 5.05 mole
= final moles of gas = 5.05 mole
Now we put all the given values in this formula, we get
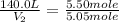
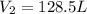
Therefore, the volume of the balloon after 45.0 hour is 128.5 L