Answer:
V₂=1.76 m³
P=222.03 KPa
Step-by-step explanation:
Given that
For tank 1
V₁=1 m³
T₁= 10°C = 283 K
P₁=350 KPa
For tank 2
m₂=3 kg
T₂=35°C = 308 K
P₂=150 KPa
We know that for air
P V = m R T
P=pressure ,V= Volume,R= gas constant ,T= temperature ,m =mass
for tank 2
P₂ V₂ = m₂ R T₂
By putting the values
150 x V₂ = 3 x 0.287 x 308
V₂=1.76 m³
Final mass = m₁+m₂
m =m₁+m₂
The final volume V= V₂+V₁
V= 1.76 + 1 m³
V= 2.76 m³
The final temperature T= 19.5°C
T= 292.5 K
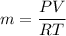

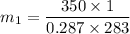
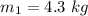
m =m₁+m₂
m =4.3 + 3 = 7.3 kg
Now at final state
P V = m R T
P x 2.76 = 7.3 x 0.287 x 292.5
P=222.03 KPa