Answer:
134.75935 seconds
Step-by-step explanation:
m = Amount of water = 240 mL
c = Specific heat of water = 4.2 J/mL °C
 = Change in temperature =
= Change in temperature =
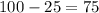
t = Time taken
P = Power = 1100 W
E = Pt
Efficiency = 51 %
So, Energy = 0.51Pt
As energy is conserved
Q = 0
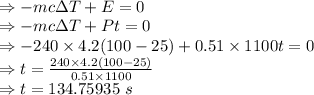
The it takes to raise the temperature is 134.75935 seconds