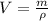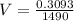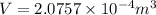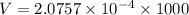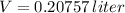Answer:
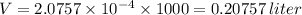
Step-by-step explanation:
Given that:
quantity of heat to be removed,
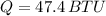
we know,
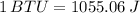
density of freon,
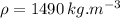
latent heat of vaporization of freon,

So,
heat to be removed in joules,
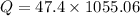
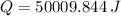
Now, the quantity of freon required to remove the above mentioned heat:
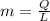
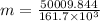
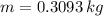
Now, volume