Answer: Option (c) is the correct answer.
Step-by-step explanation:
It is given that the scientist is claiming that all the spontaneous reactions are exothermic in nature.
And, it is known that when a reaction is spontaneous in nature then
 is negative.
is negative.
Now, the relation between Gibb's free energy, enthalpy and entropy is as follows.
 =
=
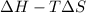
So, when a catalyst is present in a chemical reaction then we do not need to give large amount of heat from outside. And, because of this the enthalpy of reaction will not be highly positive.
Hence, the value of
 will result in a negative value which means the reaction is spontaneous.
will result in a negative value which means the reaction is spontaneous.
Thus, we can conclude that an endothermic reaction that only proceeds when a catalytst is present, would provide the strongest challenge to their claim.