Answer:
1 molecule of vanilin contains 1 methoxy group
Step-by-step explanation:
In the Zeisel method 1 mole of AgI is equivalent to 1 mole of methoxyl group.
1,64g of AgI are:
1,64g AgI×
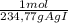 = 6,99x10⁻³ moles of AgI ≡ moles of methoxy groups.
= 6,99x10⁻³ moles of AgI ≡ moles of methoxy groups.
1,06g of vanilin are:
1,06g vanilin×
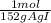 = 6,97x10⁻³ moles of vanilin
= 6,97x10⁻³ moles of vanilin
The ratio of moles of methoxy groups/moles of vanilin is:
6,99x10⁻³ moles/6,97x10⁻³ moles = 1,00
That means that 1 molecule of vanilin contains 1 methoxy group.
I hope it helps