Answer: Pairs CsCl,
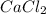 CaS,
CaS,
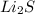 KBr,
KBr,
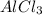 ,
,
 KI, and
KI, and
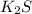 would theoretically be the better conductor of electricity.
would theoretically be the better conductor of electricity.
Step-by-step explanation:
Ionic compounds are the compounds which are readily soluble in polar solvents like water. When an ionic compound is dissolved in water then it dissociates into ions.
As electricity is the flow of ions or electrons. Therefore, ionic solutions are able to conduct heat and electricity.
For example, CsCl,
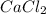 CaS,
CaS,
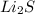 KBr,
KBr,
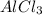 ,
,
 KI, and
KI, and
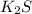 are all ionic compounds.
are all ionic compounds.
Generally, an ionic compound is formed when a metal and a non-metal chemically combines with each other.
Thus, we can conclude that pairs CsCl,
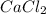 CaS,
CaS,
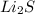 KBr,
KBr,
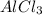 ,
,
 KI, and
KI, and
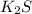 would theoretically be the better conductor of electricity.
would theoretically be the better conductor of electricity.