Answer:

This is green light
Step-by-step explanation:
According to the photoelectric effect, the maximum kinetic energy of an photoelectron is given by:
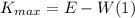
Here, E is the energy of the photon and W is the minimum energy required to remove an electron from the surface of the metal, W is defined as:
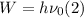
The Planck – Einstein relation states that the energy of a photon is equal to its frequency multiplied by the planck constant:
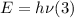
Recall that
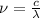 . Replacing (3) and (2) in (1):
. Replacing (3) and (2) in (1):
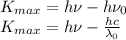
Solving for
 :
:
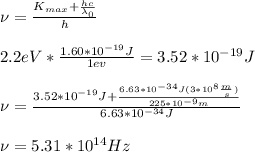
This is green light