Answer : The value of
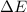 for the system is -77880 J
for the system is -77880 J
Explanation :
First law of thermodynamic : It states that the energy can not be created or destroyed, it can only change or transfer from one state to another state.
As per first law of thermodynamic,
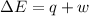
where,
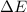 = internal energy of the system
= internal energy of the system
q = heat absorbed by the system
w = work done by the system
As we are given that:
q = 120 J
w = -78 kJ = -78000 J (1 kJ = 1000 J) (system work done by system)
Now put all the given values in the above expression, we get:
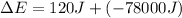
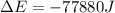
Therefore, the value of
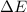 for the system is -77880 J
for the system is -77880 J