Step-by-step explanation:
The given reaction equation showing heat of combustion is as follows.
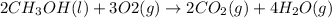 ....... (1)
....... (1)
So, for 2 mol methanol the value for heat of combustion is as follows.
2 mol x 182.6 Kcal/mol = -365.2 Kcal
Now,
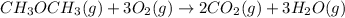 -347.6 kcal/mol
-347.6 kcal/mol
When we reverse the 2nd reaction
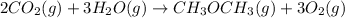 heat of reaction +347.6 kcal {sign reversed with reaction} ........ (2)
heat of reaction +347.6 kcal {sign reversed with reaction} ........ (2)
Now, adding equatio (1) and (2) we get the following.
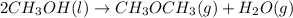
Now, heat of combustion will also be added.
+347.6 kcal +(-365.2 Kcal)
= -17.6 Kcal
Since, the reaction occurs at STP , water will be in liquid state as B.P =
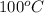
So, energy needed for water to convert to vapour = 1 mol x 10 Kcal/mol = 10 Kcal, and it will be absorbed by water to form vapor .
Hence, heat of dehydration =
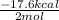
= - 8.8 Kcal/mol
Therefore, the net calorific value for this after water is evaporated to form gas = 17.6 - 10 = 7.6 Kcal
or,
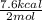
= 3.8 Kcal/mol