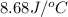Answer : The value of
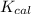 for a calorimeter is
for a calorimeter is
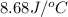
Explanation :
First we have to calculate the mass of water.
As we know that the density of water is 1 g/mL. The volume of water is 5.00 mL.

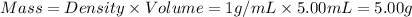
Now we have to calculate the heat lost by the hot water.
Formula used :
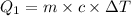
or,
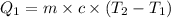
where,
Q₁ = heat lost by hot water = ?
m = mass of water = 5.00 g
c = specific heat of water =
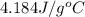
 = initial temperature =
= initial temperature =
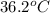
 = final temperature =
= final temperature =
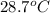
Now put all the given value in the above formula, we get:
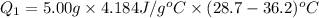
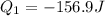
Heat lost by the hot water is 156.9 J and the negative sign indicate that the heat is lost or released.
Now we have to calculate the heat gained by the cold water.
Formula used :
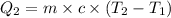
where,
Q₂ = heat gained by the cold water = ?
m = mass of water = 5.00 g
c = specific heat of water =
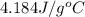
 = initial temperature =
= initial temperature =
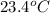
 = final temperature =
= final temperature =
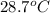
Now put all the given value in the above formula, we get:
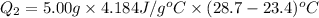
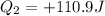
Heat gained by the cold water is 110.9 J and the positive sign indicate that the heat is gained or absorbed.
Now we have to calculate the difference between heat lost and heat gained that is equal to the heat gained by calorimeter.
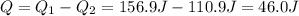
Now we have to calculate the
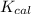 for a calorimeter.
for a calorimeter.
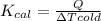
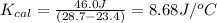
Therefore, the value of
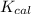 for a calorimeter is
for a calorimeter is