Answer:
ΔV = -0.97 m³/ kg
ΔH = 0 kJ/ kg
Step-by-step explanation:
To determine the change in the specific volume we need to use the ideal gas law:
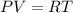
where P: pressure of the gas V: volume of the gas, R: ideal gas constant= 0.4119 kJ/kg.K = 0.4119 kPa.m³/kg.K and T: temperature of the gas.
The V₁, at a compressed pressure is:
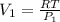
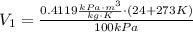
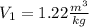
Similarly, the V₂ is:

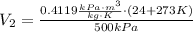
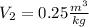
Now, the change in the specific volume because the compressor is:
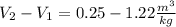
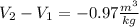
Finally, to calculate the change in the specific enthalpy, we need to remember that neon is an ideal gas and that is an isothermal process:
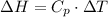
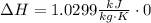
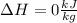
Have a nice day!