Answer:
There is 1 carbon atom in the empirical formula.
Step-by-step explanation:
First, let's use the given mass of CO₂ to calculate the moles of C, which will be the same amount of C moles present in the sample:
- 3.702 g CO₂ ÷ 44g/mol = 0.0841 mol CO₂ = 0.0841 mol C
Then with the mass of H₂O we calculate the moles of H:
- 1.514 g H₂O ÷ 18gH₂O/mol *
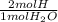 = 0.1682 mol H
= 0.1682 mol H
Now we calculate the mass of C, H in the sample, in order to calculate the mass of O, which is calculated by substracting the mass of C and H from the total mass:
- 0.0841 mol C * 12 g/mol = 1.0092 g C
- 0.1682 mol H * 1 g/mol = 0.1682 g H
- g O = 2.750 - (1.0092+0.1682) = 1.5726 g O
Then we convert g O to mol O
- 1.5726 g O ÷ 16g/mol = 0.0983 mol O
C ⇒ 0.0841 mol C
H ⇒ 0.1682 mol H
O ⇒ 0.0983 mol O
Now we divide those values by the lowest among them:
- O ⇒ 0.0983 / 0.0841 = 1.1689 ≅ 1
So the empirical formula is CH₂O
There is 1 carbon atom in the empirical formula.