Answer:
- Theoretical yield = 568.47 g BaCl₂
- Actual yield = 406.45 g BaCl₂
Step-by-step explanation:
Hydrochloric acid(aq) + Barium hydroxide(aq) → Barium chloride(aq) + Water(l)
- 2HCl(aq) + Ba(OH)₂(aq) → BaCl₂(aq) + 2H₂O(l)
For the theoretical yield of BaCl₂ we use all the initial mass of HCl, and convert it into mass of BaCl₂:
- 5.46 g HCl *
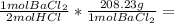 568.47 g BaCl₂
568.47 g BaCl₂
For the actual yield we multiply the theoretical yield by the percent yield:
- 568.47 g BaCl₂ * 71.5/100 = 406.45 g BaCl₂