Answer:
The total energy change for the production of one mole of aqueous nitric acid is 709,8kJ
Step-by-step explanation:
The three steps in the industrial production of nitric acid are:
4NH₃(g) + 5O₂(g) → 4NO(g) + 6H₂O(l) ΔH = −1166.0 kJ/mol (1)
2NO(g) + O₂(g) → 2NO₂(g) ΔH = −116.2 kJ/mol (2)
3NO₂(g) + H₂O(l) → 2HNO₃(aq) + NO(g) ΔH = −137.3 kJ/mol (3)
For the total process:
4NH₃(g) + 6O₂(g) + NO₂ → 3NO(g) + 2HNO₃(aq) + 5H₂O(l)
The ΔH is (Hess's law) : -1166,0kJ/mol - 116,2kJ/mol - 137,7kJ/mol = -1419,5 kJ/mol
As the total process produce two moles of nitric acid, the total energy change for the production of one mole of aqueous nitric acid is:
-1419,5 kJ/mol×
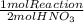 = 709,8 kJ
= 709,8 kJ
I hope it helps!