Answer:
The order will be:
pH 3.5 > pH 6.5 > pH 7
Step-by-step explanation:
The solubility equation for Mn(OH)₂ is:
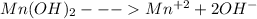
As per the reaction, Mn(OH)₂ is giving hydroxide ions
The given conditions are
a) Pure water = here the pH of water is 7.
[OH⁻] = 10⁻⁷ M
b) pH = 6.5
pOH = 7.5
[OH⁻] = 3.16 X 10⁻⁸ M
c) pH = 3.5
pOH = 10.5
[OH⁻] =3.16 X 10⁻¹¹ M
Thus from the three solutions and water the maximum concentration of hdyroxide ion is in pure water, hence the Mn(OH)₂ will be least soluble in it.
More the concentration of hydroxide, lesser the solubility of Mn(OH)₂
Order will be
pH 3.5 > pH 6.5 > pH 7