Answer:
1) The blood buffering system maintains the pH of blood near 7.4, as long as it must be quite neutral to favor metabolism.
2) The blood buffering system uses the H2CO3 / HCO–3 conjugate acid/base pair because such relationship in the Henderson–Hasselbalch equation is roughly one.
3) It is facilitated by the enzyme carbonic anhydrase, which interconverts carbon dioxide and water to carbonic acid.
Step-by-step explanation:
Hello,
In this case, the three statements accurately describing the blood buffering system in humans are:
1) The blood buffering system maintains the pH of blood near 7.4, as long as it must be quite neutral to favor metabolism.
2) The blood buffering system uses the H2CO3 / HCO–3 conjugate acid/base pair because such relationship in the Henderson–Hasselbalch equation is roughly one.
3) It is facilitated by the enzyme carbonic anhydrase, which interconverts carbon dioxide and water to carbonic acid as follows:
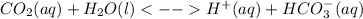
Best regards.