Answer : The value of the scaling factor by which all reagents reduced must be 0.0711
Explanation :
First we have to calculate the molecular weight of alum.
The formula of alum is,
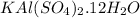
Molecular weight of Alum =
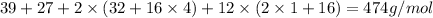
Now we have to calculate the moles of alum.
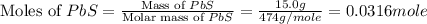
Now we have to calculate the moles of aluminium.
From the molecular formula of the alum we conclude that, 1 mole of alum constitutes 1 mole of aluminium.
So, 0.0316 moles of alum constitutes the number of moles of aluminium = 0.0316 moles
Now we have to calculate the mass of aluminum.
Molar mass of aluminium = 27 g/mole
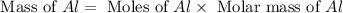
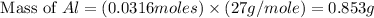
Now we have to calculate the value of the scaling factor.
In the the original procedure, the amount of aluminium required = 12.0 g
The amount of aluminium required to produce 15 gram alum = 0.853 g
The scaling factor =
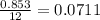
Therefore, the value of the scaling factor by which all reagents reduced must be 0.0711