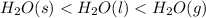Answer : The order of increasing standard molar entropy is:
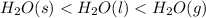
Explanation :
Entropy : It is defined as the measurement of randomness or disorderedness in a system.
The order of entropy will be,
As we are moving from solid state to liquid state to gaseous state, the entropy will be increases due to the increase in the disorderedness.
As we are moving from gaseous state to liquid state to solid state, the entropy will be decreases due to the decrease in the disorderedness.
From this we conclude that the solids have less disorder in the system than liquids because the molecules in the solid are closely or tightly packed as compared to liquid.
The liquids have less disorder in the system than gases because the molecules in the liquid are more close as compared to gases.
That means the more disorderedness in a system, the more will be the entropy.
So, the order of increasing standard molar entropy is: