Answer:
A substance consisting of 3.7 grams of beryllium fluoride would be equal to how many moles of beryllium fluoride?
How many grams of sodium are equal to 1.5 moles of sodium?
Step-by-step explanation:
The molar mass is related to number of moles and mass of an element or compound.
Using the expression:
Number of moles =
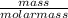
For any problem, where the number of moles is known, we can find the mass.
A substance consisting of 3.7 grams of beryllium fluoride would be equal to how many moles of beryllium fluoride?
Here we know the mass, we simply use the molar mass to find the number of moles.
How many grams of sodium are equal to 1.5 moles of sodium?
Here number of moles is given, we use the molar mass to find the mass of the sodium given