Final Answer:
The structural formula of the compound with the condensed structural formula CH₃C(CH₃)₂C(CH₂CH₃)₂CH₂CH₂CH₂CH₂CH₃ is shown below:
```
H
|
CH₃C
|
CH₃
|
C
|
CH₃
|
C
|
CH₃
|
C
/ \
CH₃ CH₂CH₃
|
C
|
CH₃
|
CH₂
|
CH₃
|
CH₂
|
CH₂
|
CH₂
|
CH₃
```
Step-by-step explanation:
The given condensed structural formula CH₃C(CH₃)₂C(CH₂CH₃)₂CH₂CH₂CH₂CH₂CH₃ represents a branched hydrocarbon with multiple methyl
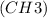 and ethyl (CH₂CH₃) groups. To construct the structural formula, it is essential to interpret the condensed notation by identifying the different carbon atoms and their attached groups. The methyl groups (CH₃) are represented by single carbon atoms, while ethyl groups (CH₂CH₃) consist of two carbon atoms connected by a single bond.
and ethyl (CH₂CH₃) groups. To construct the structural formula, it is essential to interpret the condensed notation by identifying the different carbon atoms and their attached groups. The methyl groups (CH₃) are represented by single carbon atoms, while ethyl groups (CH₂CH₃) consist of two carbon atoms connected by a single bond.
Starting from the left, the first carbon atom has three hydrogen atoms and one methyl group attached. Moving to the second carbon, it is bonded to two methyl groups and one ethyl group.
The ethyl group is then connected to another ethyl group through a single bond. The third carbon is part of both ethyl groups, and the fourth carbon is bonded to one ethyl group and two hydrogen atoms. The rest of the carbon atoms follow, forming a chain of ethyl groups connected by single bonds.
The structural formula provides a detailed representation of the spatial arrangement of atoms in the compound, offering insights into its geometry and connectivity.