Answer:
d = 9.08 Kg/m³
Radon concentrations are greater in the basement.
Step-by-step explanation:
To find the density of radon, we need to use the ideal gas law:
 (1)
(1)
where, P: pressure, V: volume, n: moles of gas, T: absolute temperature y R: ideal gas constant.
Knowing that the number of moles is:
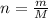 (2)
(2)
where m: mass of gas, and M: molar mass of gas,
Then, we can replace the number of moles into equation (1):
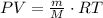
The density of the gas is giving by:
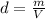 (3)
(3)
where: m: mass and V: volume of gas
Now, we can calculate the density of Radon:
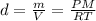
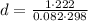
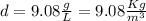
The ²²²Rn is produced by the decay of radium isotope ²²⁶Ra from the uranium-238 decay chain. Uranium is present in ground minerals, from which the radon gas can emerge and then accumulate in basements of buildings, due to its high density. Hence, because of its high density compared to the air (about 1.225 Kg/m³), radon concentrations are likely to be greater in the basement than on the top floor of a building.