Answer:
2,25mg of Fe²⁺
Step-by-step explanation:
A chemical concentration is defined as an amount of solute per amount of solution. The diluted solution has an iron (II) concentration of 1,35x10⁻³ mg/mL. As the dilution was of 15 mL in 100 mL, the concentration of the original solution is:
1,35x10⁻³ mg/mL ×
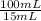 = 9,00x10⁻³ mg/mL
= 9,00x10⁻³ mg/mL
As the total volume of the concentrated iron solution is 250 mL, the total mass of this solution is:
250 mL ×
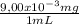 = 2,25 mg of Fe²⁺
= 2,25 mg of Fe²⁺
I hope it helps!