Answer:
2.572 g
Step-by-step explanation:
From the question;
Volume of Helium gas, V is 18.5 L
Pressure,P is 85.5 kPa
Temperature, T is 296 K
We are required to calculate the mass of Helium gas
Step 1: Calculate the number of moles of Helium gas
- This can be done using the ideal gas equation.
- The ideal gas equation is PV=nRT, where P is the pressure, V is the volume, n is the number of moles, T is the temperature and R is the ideal gas constant which is 8.314 kPa.L/K.mol
Rearranging the formula;
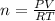
Thus,
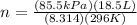
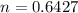
n = 0.643 moles
Therefore, moles of Helium are 0.643 moles
Step 2: Calculate the mass of Helium gas
To calculate the mass we multiply the number of moles by the molar mass.
Mass = Number of moles × Molar mass
Molar mass of Helium = 4.00 g/mol
Therefore;
Mass of He = 0.643 moles × 4.00 g/mol
= 2.572 g
Hence, mass of helium gas is 2.572 g