Answer:
a) The half life of the element is 231 days.
b) It is going to take around 31.5 days for a sample of 100 mg to decay to 91 mg.
Explanation:
The radioactivity of the sample can be modeled by the following exponential equation:
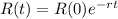
In which t is the time in days, r is the decay rate and
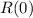 is the initial radioactive percentage.
is the initial radioactive percentage.
We have that:
In 440 days the radioactivity of a sample decreases by 74 percent.
This means that
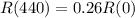 .
.
This helps us find r.
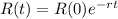
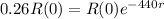
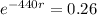
Applying ln to both sides.
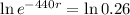
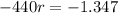
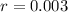
(a) What is the half-life of the element?
This is t when
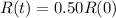
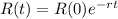
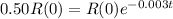
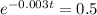
Again, we apply ln to both sides of the equality.
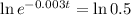
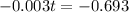
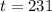
The half life of the element is 231 days.
(b) How long will it take for a sample of 100 mg to decay to 91 mg?
This is t when
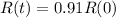
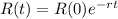
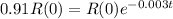
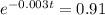
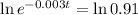
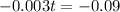
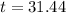
It is going to take around 31.5 days for a sample of 100 mg to decay to 91 mg.