a. 2 M
b. 1.5 M
Further explanation
Given
a 4 moles in 2 dm of solution?
b 0.3 moles in 200 cm3 of solution
Required
The concentration of a solution
Solution
Molarity shows the number of moles of solute in every 1 liter of solution or mmol in each ml of solution
Can be formulated :
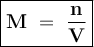
a.
moles(n) = 4
volume(V)=2 L solution
M = 4 : 2 = 2
b.
moles(n)=0.3
volume(V)=200 cm3 = 0.2 L
M = 0.3 : 0.2 = 1.5