Answer:
a. 2.29
b. 1.56
Step-by-step explanation:
a. For the reaction given, let's make an equilibrium chart. But first, let's calculate the initial concentration of iron(III) chloride hexahydrate. The molar masses are:
Fe = 55.8 g/mol
H = 1 g/mol
O = 16 g/mol
Fe(H₂O)₆ = 55.8 + 6*2*1 + 6*16 = 163.8 g/mol
n = mass/ molar mass
n = 0.7438/163.8
n = 0.0045 mol
The initial concentration is n/V(L) = 0.0045/0.2 = 0.0225 M
[Fe(H₂O)₆]³⁺(aq) ⇄ [Fe(H₂O)₅OH]²⁺(aq) + H⁺(aq)
0.0225 0 0 Initial
-x +x +x Reacts ( stoichiometry 1:1:1)
0.0225 - x x x Equilibrium
Ka is the equilibrium constant, and:
Ka =
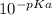
Ka =
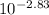
Ka = 1.479x10⁻³
And
![Ka = ([H^+]*[[Fe(H2O)5OH]^(+2)])/([[Fe(H2O)6]^(+3)])](https://img.qammunity.org/2020/formulas/chemistry/college/infepv5b3pkxls2z02lejjpm0zxf69b88h.png)
1.479*10⁻³ = (x*x)/(0.0225 - x)
1.479*10⁻³ = x²/(0.0225 - x)
x² = 3.32775*10⁻⁵ - 1.479*10⁻³x
x² + 1.479*10⁻³x - 3.32775*10⁻⁵
By Bhaskara's equation:
Δ = (1.479*10⁻³)² - 4*1*(-3.32775*10⁻⁵)
Δ = 1.353x10⁻⁴
x = (-1.479*10⁻³±√1.353x10⁻⁴)/2
x must be positive, so let's ignore the negative value:
x = (-1.479*10⁻³ +√1.353x10⁻⁴)/2
x = 5.076*10⁻³
So, [H⁺] = 5.076*10⁻³
pH = -log[H⁺]
pH = -log(5.076*10⁻³)
pH = 2.29
b. If the iron(III) concentration reduces to 1.0x10⁻¹⁰ M, it means that the reaction with the hydroxide consumed:
0.0225 - 1.0x10⁻¹⁰ = 0.022499 M which will form more iron (II) and H⁺. So the new concentration of iron (II) and H⁺ will be = 0.022499 + 5.076*10⁻³ = 0.02758 M
So, pH = -log[H⁺]
pH = -log0.02758
pH = 1.56