Answer:
0.70 M
Step-by-step explanation:
- Molarity is the concentration of a given solution in moles per liter.
- Molarity is given in moles per liter of solution.
- It is calculated by dividing the moles of solute by the volume of solution.
In this case;
We are given;
Number of moles of HCl = 3.5 Moles
Volume of solvent, H₂O is 5.0 L
But,
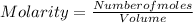
Therefore;
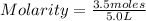
= 0.70 M
Thus, the molarity of the solution is 0.70 M or 0.70 moles/liter