Answer:
0.255 moles
Step-by-step explanation:
- The relationship between moles, mass and the molar mass of a compound is given by;
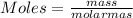
- Molar mass of a compound is equivalent to the relative formula mass of a compound that is calculated by adding the atomic masses of atoms making the compound.
In our case.
We are given the compound PCl₃ whose;
Mass is 35 g
Molar mass is 137.33 g/mol
But;
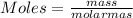
Therefore;
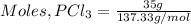
= 0.255 moles
Therefore, 35 g of PCl₃ contains 0.255 moles