Answer:
Energy of a photon is
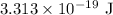
Step-by-step explanation:
Given:
Frequency of the photon is,
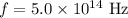 .
.
Energy of a photon of frequency
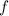 is given as:
is given as:
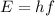 , where,
, where,
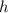 is called Planck's constant.
is called Planck's constant.

So, energy of a photon is:
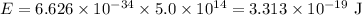
Therefore, the energy of a photon whose frequency is
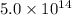 Hz is
Hz is
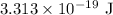 .
.