The mass of one mole of a hydrogen atom is 1 which weights 1.008 grams.
Step-by-step explanation:
Mole is defined as the base unit of amount of substance in the system international, whereas mass is both a measure of its resistance to acceleration and the property of the physical body — the basic unit of mass in kilograms.
To know the mass of an atom, we need a periodic table and Avogadro’s number of
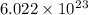 .
.
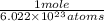
=
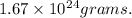
So, the value of one mole of a hydrogen atom is 1.