Answer:
26.16 moles of water
Step-by-step explanation:
As per balanced equation given:
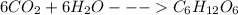
Six moles carbon dioxide will react with six moles of water to give one mole of glucose
As per problem the moles of glucose produced = 4.36
so the moles of water required is = 6X4.36 =26.16 moles
the same number of moles of carbon dioxide will also be required.