Answer:
The volume of the quantity of gas when the pressure changes to 740 mm Hg is approximately 9.92 L
Step-by-step explanation:
The properties of the quantity of gas are;
The initial pressure of the gas, P₁ = 1.39 atm
The initial volume of the given volume of gas, V₁ = 6.95 L
The final pressure of the quantity of gas, P₂ = 740 mmHg
Boyle's Law states that at constant temperature, the pressure, P, of a given mass of gas is inversely proportional to its volume, V
Mathematically Boyle's Law can be written as follows;
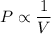
Therefore, we have;
P₁·V₁ = P₂·V₂
Where;
V₂ = The final volume of the quantity of gas when the pressure changes to 740 mm Hg
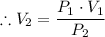
By substituting the known values, we have;
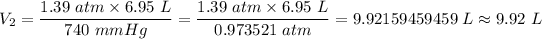
The final volume of the quantity of gas when the pressure changes to 740 mm Hg = V₂ ≈ 9.92 L