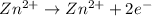Answer:
The oxidation occur on the zinc electrode.
Step-by-step explanation:
An electrochemical cell is a device in which chemical energy produced during reaction is converted into electrical energy. in these type of cells oxidation occurs at anode and reduction occurs at cathode.
At cathode :(reduction)
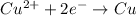
At anode: (oxidation)