Answer:
21 milliliters of nitric acid.
Explanation:
Given:
Volume of the solution is,
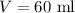
Percentage of nitric acid in the solution is 35 % of

Therefore, volume of nitric acid in the solution is =
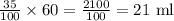
Hence, the solution contain 21 milliliters of nitric acid.