Answer : The reactant Kl is an ionic compound.
Explanation :
Ionic compound : It is defined as the compound which is formed when electron gets transferred from one atom to another atom.
Ionic compound are usually formed when a metal reacts with a non-metal.
Potassium iodide is an ionic compound because potassium element is a metal and iodide element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
The charge on potassium is (+1) and the the charge on iodide is (-1). Thus, the formula of the compound potassium iodide will be
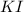 .
.
Hence, the reactant Kl is an ionic compound.